Cryogenics is the branch of physics dealing with the behavior of matter at very low temperatures. The origin of the word cryogenics comes from the Greek words ‘cryo’ and ‘genic’ meaning “frost” and “to produce”. Areas under cryogenics include cryobiology, cryonics, cryoelectronics, and cryotronics. In the industrial field cryogenics exposes metals and other material to the very low temperatures to achieve enhanced crystalline structure, improve wear resistance, increased dimensional stability, and decreased residual stress.
According to the laws of thermodynamics, the lowest temperature that can be achieved is absolute zero or 0oK at absolute zero molecules stop moving. Absolute zero has never been reached but scientists have reached a few billionths above absolute zero.
Cryogenics principles can also be used to liquefy gasses at extremely low temperatures.
Cryobiology is the study of living organisms at extremely low temperatures. The principles of cryobiology are used by cyropresevation which is the process of removing living cells, freezing them, storing them, and later reintroducing them into the body. Organisms frozen under cryobiology include bacteria, plants, and animals (both vertebrates and invertebrates). Using cryogenics to freeze entire body parts is known as cryonics.
Cryoprotectants are chemicals usually sugars that are used to prevent freezing in the cells during cryopreservation. This process is called virtification. The vertified cells are undamaged during the freezing process because the cryoprotectant protects the cells while they are under the freezing conditions. The common sugars used as cryoprotectants chemicals usually sugars that are used to prevent freezing in the cells during cryopreservation therefore increasing the organism’s viability. The common sugars used as cryoprotectants ethylene glycol, glycerol, propylene glycol, sucrose, trehalose, and dimethyl sulfoxide. Different cryoprotectants should be used depending on the types of cells being frozen since some cryoprotectants can be toxic to the different types of cells.
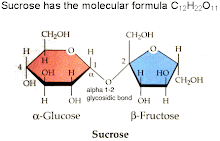
Sucrose:
Sucrose (C12H22011) is often known as the most abundant pure organic chemical. It is formed when fructose and glucose combined with an acetal oxygen bridge. The structure of the sucrose is crystalline. Sucrose is a carbohydrate that is very common in most fruits and vegetables. The sucrose from the fruits and vegetables are the major products of photosynthesis. Sucrose can also be described as a disaccharide formed by the joining of two monosaccharides. Since sucrose is a non reducing sugar it is considered an unusual disaccharide. This is true due to the removal of the hydrogen atoms during dehydration which closes the chemical ring and is now unable to open. Sucrose is water soluble in water and has a caramel odor.
Sucrose should be stored in a sealed container that is stored in cool dry place that is well ventilated and away from any ignition sources. Sucrose can be considered flammable if powder is dispersed through the air at high concentrations in the presence of an ignition source. Sucrose is considered stable under normal storage and use. In use sucrose usually comes as a fine white powder.
Sucrose can be used as a cryoprotectant because when it a partial dehydrate it prevents ice crystals from forming in the cells. In plants that are freeze tolerant sucrose is the most common sugar. When the plant feels the very low temperatures they can increase their sucrose levels ten fold.
Cooling Baths:
Cooling baths are used vey often in organic chemistry. To make a cooling bath to a temperature down to about -40o Celsius involves mixing slurry of ice with an inorganic salt.
Crush the ice with the salt using a mortar and pestle this will make the slurry and mix in the ice. These salts include sodium chloride and sodium chloride. Baths must be watched closely to ensure temperature stays at desired range.
When making cooling baths at very low temperatures Dewar flasks can be used to keep the bath at desired temperature. Dewar flasks maintain temperature because they are made of two pieces of metal with vacuum in between them. This prevents the cold transfer into the air. Dewar flasks can be used for both very hot and very cold liquids.
To make baths below -40oC an organic solvent is poured over dry ice slurry. Liquid nitrogen can be used to reach temperatures below -100oC.
Water Resistant Pen Shape Stem Thermometer:
The thermometer has a measuring range from -50oC to 200oC. It has an accuracy of 1oC between -19.9oC and 119.9oC, 2oC between -50oC and -20oC, 2oC between 120oC and 199.9oC, and 3oC between 200oC and 300oC.
Water resistant pen shape stem has water resistance of IP65. It uses a stainless steel probe.
The thermometer should be washed after each use. Once the display screen becomes dim batteries should be replaced. After replacing batteries make all screws are tight. It uses one 1.5 volt, type A76 battery or equivalent.
Thermometer should not be used in extreme environmental temperatures. To record a measurement probe the thermometer into test substance about 25mm/ 1” minimum and let reading stabilize.
Sodium Chloride:
Sodium chloride (NaCl) is a white odorless crystalline compound. Slightly toxic if ingested. Sodium chloride is often referred as common table salt. It is created from the sodium and chlorine atoms forming an ionic bond. Sodium chloride is not combustible. When storing it should be stored with acetates, halides, sulfates, sulfites, thiosulfates, and phosphates the area the chemical is being stored should be cool and dry. The chemical should also be kept in a container with a tight lid. Sodium chloride is soluble in water and glycerol, but only slightly soluble in alcohol. It is not soluble in most other liquids.
Sodium chloride is produced mainly by evaporating any body of water that has salt. It is also produced by the mining rocks since it is a mineral halite.
It is often used for seasoning and as a food preservative. Sodium chloride can also be used to deice roads. In the ocean sodium chloride provides for most of the salinity.
Calcium Chloride:
Calcium chloride (CaCl2) is a non-hazardous chemical and not a threat to the environment. Calcium chloride is made by the bonding of one calcium ion and two chlorine ions. It comes in powder, crystals, and flakes and can be toxic if ingested. It behaves as an ionic halide under normal conditions. Calcium chloride is non-flammable and non-combustible solid. When storing it should be stored with acetates, halides, sulfates, sulfites, thiosulfates, and phosphates the area the chemical is being stored should be cool and dry. Calcium chloride as dihydrate is water soluble.
It can be used to deice roads and control dust. Calcium chloride can retain moisture long periods and has a very low melting point compared to most rock salts. Since it absorbs water very quickly it is used to dry gasses by passing the gasses through the chemical. It is also used as a drying agent form many other materials.
Aquariums:
When cleaning a tank never use alcohol, ammonia, bleach, glass cleaner, or soap. To clean a tank use tap water and a sponge. After filling the tanks with tap water they should be left alone form two weeks to remove all the chlorine. Dechlorinator can be used to speed up the process or removing chlorine. To prevent ammonia build-up change 10% of the tanks water every month. For a fresh water tank the pH should be 7. pH should be checked weekly. Tanks should receive plenty of natural light.
Tanks should have 1 inch of gravel so bacteria can grow and convert toxic ammonia into NO2 and NO3. The oxygen level of the tank should be 8 parts per million. If tank has too much bacteria the bacteria will use up all the oxygen and the living organisms will die. Too much decaying matter will also cause an oxygen shortage in the tank.
Cloudy water means too much bacteria is in the tank and the tank should be aerated, 20% of its water should be changed, and the excess food or decaying matter should be removed.
Toxins are removed from the tank by the activated charcoal in the filter. The filter uses mesh to remove debris. The filters should be changed weekly and the activated charcoal replaced monthly. Algae build-up on rocks, gravel, and sides of the tank should be removed weekly.
Elodea:
Elodea is a member of the Plante Kingdom
Often Hydrilla and Egeria plants are confused with elodea plant because elodea has become a synonym for many small submerged plants that have leaves that look like grass. The plant is rooted and perennial. The plant can also survive and grow as floating fragments. The elodea plant has multiple branches. The leaves of the elodea plant are in whorls of three. Each leaf has finely toothed margins. The stems of the elodea are slender and round in the cross section they can grow to be 40” long. The flowers have three white petals with a protective wax coating. The plant forms winter buds that form at the stem tips at the bottom of the water.
Elodea lives in nutrient rich water such as ponds or lakes. The water is often hard. The elodea usually forms large masses in the water.
Elodea reproduces by seed and vegetatively. The vegetative method is much more common and occurs when the stem of the plant fragments. Less often a male flower will open spreading pollen on the top of the water until it reaches a female flower. Since the pollen doesn’t always reach the female flower due to outside factors such as weather and interference of other organisms this method of reproduction is very rare.
Elodea has many uses for people with aquariums. Elodea is often used to oxygenate aquariums. It can also be used as shelter for small fishes and aquatic invertebrates.